Inducing Evolution in Baker’s Yeast – Saccharomyces cerevisiae
L. Van Warren
Warren Design Vision
10/30/2005
Introduction
Saccharomyces cerevisiae, or budding Baker’s yeast is Latin for “Sugar Fungus Beer”. It converts a carbon source such as sugar and water into carbon dioxide and ethanol. Budding yeast can also feed on acetate, glycerol and other carbon sources. Note all images are hot-linked to their respective sources.
One yeast cell can ferment approximately its own weight of glucose per hour, giving rise to large volumes of CO2. Four billion yeast cells will fit in a sugar cube. That is about four million yeast cells in a cubic millimeter, about the size of a sugar crystal.
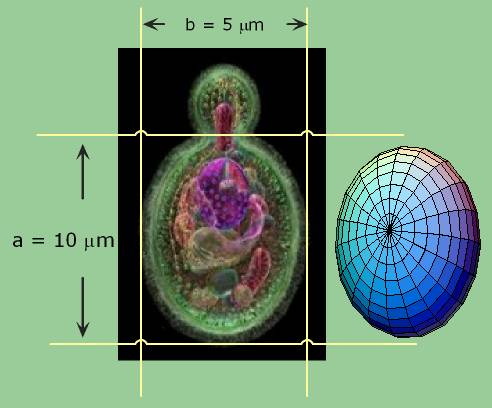
The volume of an ellipsoid is:
4/3 p abc
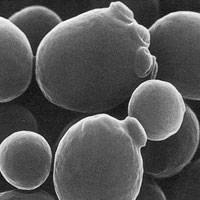
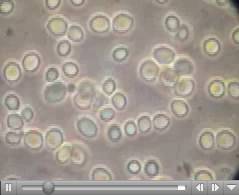
where a, b, and c are the lengths of the principal axes of the yeast. This works out to about 1000 cubic microns per yeast cell. A micron is one millionth of a meter. A centimeter is one hundredth of a meter, so you can fit four billion yeast cells in a cubic centimeter, the size of a sugar cube and we have proved our assertion. Yeast are visible under a light microscope where their growth via budding can be directly observed. This image is hot-linked to video of yeast growing.
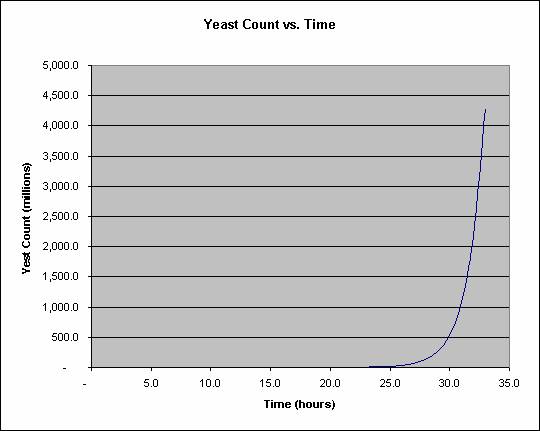
Yeast divide once per hour until the nutrients are exhausted. This sounds slow but exponential growth more than compensates for the slow speed. Each daughter cell buds off, and those buds grow and so on. Assuming fresh sugar and water are provided, it takes 23 hours to grow a cubic millimeter of yeast, a visible colony, but only 33 hours to grow 1000 times that, a cubic centimeter of yeast. This is plotted below:
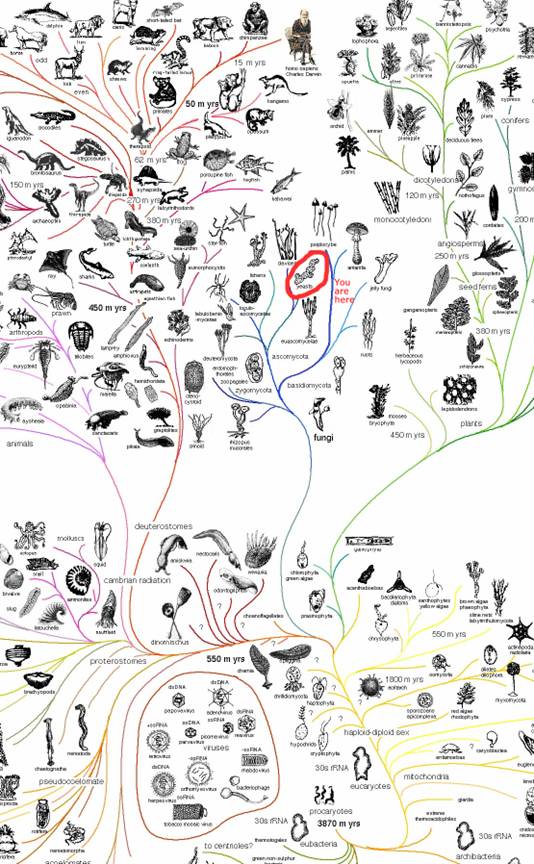
Phylogeny
According to the NCBI taxonomy browser yeast and humans diverge fairly early. Here is a list of yeast with E. coli, roundworm, frog, cow, mouse, rat and fruitfly. Matching the Latin names is an exercise for the interested reader.
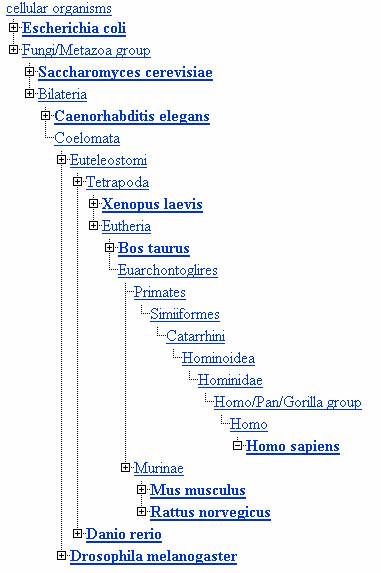
In evolutionary time, yeasts are more recent than bacteria. Yeast are 450 million years old and bacteria are 3.5 billion years old, depending on who you talk to. The yeast is in the fungi family ascomycota.
Genome
Brewer’s yeast contains up to 4 copies each of 16 chromosomes. They very rarely
recombine by mating with a separate brewing strain to create a crossbred strain
or hybrid. Brewer’s yeast is extremely genetically stable in terms of sexual
reproduction. They normally reproduce themselves by making exact duplicates of
themselves. They are members of a far ranging
genetic family.
Metabolism
We depend on yeast for all fermented wines, beers, ales. Yeast is the leavening in all breads that rise.
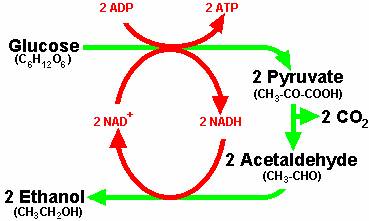
Pathways
Each of the chemical reactions that make yeast metabolism and reproduction possible are made possible by interaction with specific proteins, coded by the yeast genome. These genes, proteins and interactions have been cataloged. The interactions are diagrammed in systems like BIND as follows.
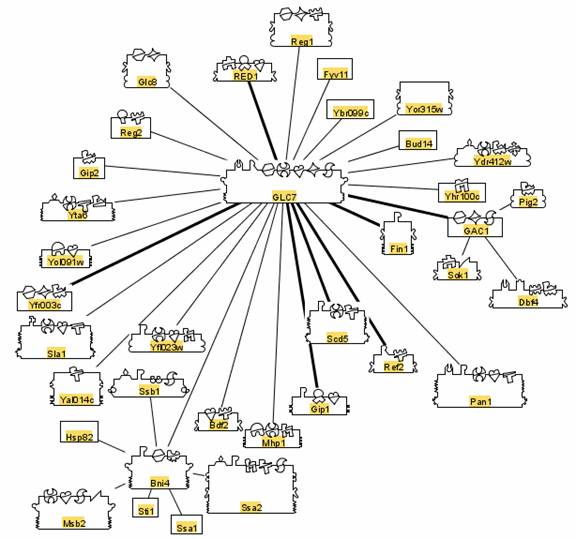
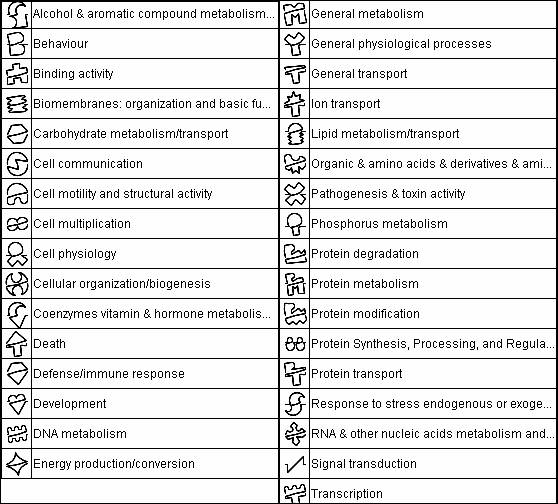
Each symbol on each protein is called a glyph. Each glyph is a code for the jobs the protein does.
Inducing Evolution
There are four ways to induce mutations. These are summarized in the graphic below. Of these ways of inducing mutations, radiation induced are the safest and fastest to execute.
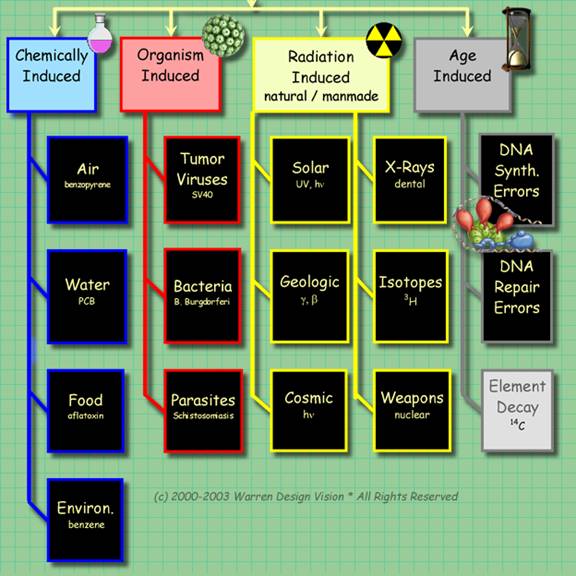
Organisms can be exposed to ultraviolet light, or safe sources of ionizing radiation, such as the sensing chamber of a smoke detector.
In a more advanced version of this experiment it might be desirable to enable recombination of yeast genes with those of other organisms. Doing so would require DNA extraction from donor organisms and digestion with restriction enzymes to make new genes available for recombination. Since I am not an expert on such methods, I will not comment further.
Once a schedule for controlled mutation is developed, control and mutant organisms can be placed in an obstacle course that simulates natural selection.
The Obstacle Course
Yeast are quite complex, but if we take a pure input/output point of view the yeast can be assessed by their tolerance of extremes in pH and in their resistance to alcohol toxicity. A 96 well culture plate can be used to expose the yeast to extremes of pH and alcohol content in carefully controlled increments.
A hand-pipette is used to transfer successive dilutions of acetic acid (vinegar), sodium hydroxide and ethanol to the control and irradiated cultures. Disposable tips should be changed each time the diluent’s type is changed. The yeast cultures should not be cross contaminated.
Quantitive concentration of yeast within the dilution ranges can be assessed by incubating the culture under appropriate temperature and humidity conditions and observing the degree of yeast growth. This can be assessed visually, or more reliably by the measuring opacity of the wells of the control and test specimens. For reproducibility, identical samples can be run in alternating rows or columns.