|
10/08/99
|
Drs. Raney/Drake
|
Biochemistry5104
|
L. Van Warren
|
1. Blood Clotting
The circulatory system must be self-sealing; otherwise continued loss of blood from even the smallest injury would be life threatening.
Normally, in a process called hemostasis, "blood unmoving", is accomplished through a cascade of events.
1) platelets adhere to damaged blood vessels to form a plug.
This is mediated by von Willebrandt factor, a glycoprotein of 225 kD in mass:
The protein binds to a specific receptor on the platelet membrane AND to the collagen and other components of the subendotheials membrane. Then coagulation occurs. A blood clot (thrombus) forms by a cascade of proteolytic reactions involving at least 20 different substances. Most are liver-synthesized plasma glycoproteins.
PurposeAbbreviation
PurposeThe soluble form of fibrin. Fibrinogen Blood Clot The precursor of thrombin Prothrombin Catalyses conversion of Fibrinogen to Fibrin Thromboplastin Ca2+ Proaccelerin Proconvertin Antihemophilic Factor Christmas Factor Stuart Factor Plasma thromboplastin antecedent (PTA) Hageman Factor Prekallikrien High MW kininogen (HMK)
2) Coagulation takes place via the following pathway.
A. Fibronogen and Its Conversion to Fibrin
Blood clots consist of arrays of cross-linked fibrin that form an insoluble fibrous network. Fibrin is made from the soluble plasma protein fibrinogen (factor I) through a proteolytic reaction catalysed by the serine protease thrombin. Fibrinogen comprises 2 to 3% of plasma protein.
Thrombin cleaves the Arg-X peptide bond where X is a Glycine in most species, enabling fibrinogen to polymerize into fibers.
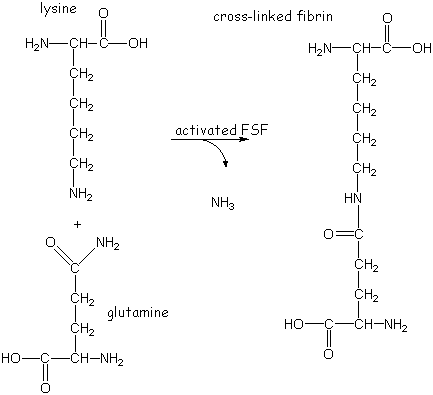
Fibrin-Stabilizing Factor (FSF) or Factor XIIIa then stabilizes fibrin clots by cross linking.
|
Fibrin-Stabilizing
Factor (FSF) Factor XIIIa
(Example shown is XIII transglutaminase) |
FSF joins the C-terminal segments of adjacent gamma chains by forming isopeptide bonds between the side chains of a glutamine on one gamma chain and a lysine on another.

The alpha chains are similarly cross-linked but at a slower rate. FSF deficient individuals tend to bleed.
Ca2+ is essential to several stages of the blood clotting cascade.
B. Thrombin Activation and the Function of Vitamin K
Human thombin is synthesized as a 579 residue prothombin II which is activated by two proteolytic cleavages catalysed by activated Stuart factor Xa the product of the preceding step of the clotting cascade.
Vitamin K Is an Essential Cofactor in the Synthesis of gamma-Carboxyglutamate.
Prothrombin, as well as the homologous factors 7, 9, and 10, is synthesized in the liver in a process that requires an adequate dietary intake of vitamin K. Lack of vitamin K or the presence of a competitive inhibitor such as dicoumarol, or warfarin, causes the production of an abnormal prothrombin that is activated by factor Xa at only 1 to 2% of the normal rate. NMR studies have established that normal prothrombin contains gamma-carboxyglutamate (Gla) residues, 10 of which occur between resdies 6 and 32 in human prothrombin. Abnormal prothrombin contains Glu in palce of these Gla residues. Vitamin K must therefore be a cofactor in the posttranslational conversion of Glu to Gla.
Vitamin K has two competitive inhibitors, Dicoumarol and Warfarin.
Vitamin K is a cofactor in the post translational converstion of glutamate to gamma carboxyglutamate (gla).
Prothrombin Activation Is Accelerated in the Presence of Factor Va, Ca2+ and Phospholipid
Physiological prothrombin activation normally takes place at a significant rate only in the vicinity of an injury. Ca2+ is required for either prothrombin or Factor Xa to bind to phospholipid membranes; These proteins are achnored to the membrane via Ca2+ bridges. The gla side chains, which are much stronger Ca2+ chelators than glu form the proteins' Ca2+ binding sites.
Clot formation is self-limiting, a safeguard that prevents clots from propagating away from the site of injury.
Thrombin Structurally Resembles Trypsin